The Impact of The Novel Food Deadline on The UK CBD Industry
Posted by Lewis Olden on
*Scroll down to the bottom of the article to find your discount code for ALL Cannacares CBD Products
Even though the UK has now left the European Union, with the transition period now completed. The European Commission’s decision regarding the Novel Food status of cannabidiol, more commonly referred to as CBD. The impending regulation means that all CBD manufacturers, producers and brands will have to ensure that all of their CBD ingestible products are registered under a Novel Food application which must be submitted to the Food Standards Agency (FSA).
It is anticipated that the CBD and Cannabis sectors across Europe will grow exponentially over the next number of years. The UK CBD industry has an opportunity to flourish and is the first nation in the European region to have an active OTC CBD market.
The FSA’s deadline for CBD producers, manufacturers and brands to submit their Novel Food application is March 31 2021. Those who do not submit an application that is not subsequently approved, will no longer be able to sell ingestible CBD products within the UK.
What is a Novel Food?
Under European Law a Novel Food is a substance that wasn’t consumed on a widespread level prior to May of 1997. Novel Foods include new products such as phytosterols and phytosterols that are ingredients in condiments that are designed to lower cholesterol. The Novel Food legislation also pertains to ingredients that may have been consumed around the world but are nascent to the European market.
An ingredient can be deemed a Novel Food if the process in which it is produced was not routinely used before 1997.
CBD oils and CBD capsules will be considered to be novel, as will CBD gummies, CBD sprays and CBD wafers. However, there are a number of ingredients that are derived from cannabis that are not included in the ruling of CBD as a Novel Food, these include hemp seed oils and hemp seeds. Hemp seed products are not included as the European market has a history of consuming hemp seeds that predates 1997.
The European Commission announced its decision in January of 2019 that CBD extracts and isolates would be listed under the Novel Food category. Cannabinoids such as CBG are also considered to be a Novel Food. At the time of writing, there is still no certified CBD product with a Novel Food licence.
The Novel Food Application
The FSA informed the UK CBD market that companies must hold a validated Novel Food application for all ingestible CBD products by the 31st of March 2021. The validation of a company’s application is the initial step towards achieving full compliance.
The Novel Food application a company submits must illustrate that each individual product is safe to use and is compliant with the Novel Food rules. Ingestible CBD products that are manufactured in the same manner using the same process can be encompassed within the same Novel Food dossier. For instance, CBD oils with 3 different flavours can be included on a single dossier. However, each product category requires its own stability data to prove the safety of the product. Novel Food applications require 5 batches of a product to be manufactured to illustrate the safety and consistency of the product for dossier submitted to the European Food Standards Agency (EFSA).
What Happens to CBD Products without Novel Food Approval?
It now seems highly unlikely the vast proportion of existing ingestible CBD products will gain Novel Food approval prior to the FSA’s deadline of 31 March 2021. However, those selling ingestible CBD products will require a validated Novel Food application. Those who do not have a validated Novel Food application for their ingestible products will risk having their stock removed from the EU & UK markets.
Alternatives to Ingestible CBD Products
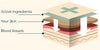
CBD patches are an incredible solution to the Novel Food problem. CBD patches deliver CBD using transdermal technology, meaning that the CBD bypasses your liver and digestive system. This means that a higher proportion of the CBD actually enters your bloodstream providing a long-lasting effect. If the effect of a CBD patch is not to your liking you can simply remove it from your skin. CBD patches provide a standardised dose of CBD unlike CBD oils.
CBD moisturisers can be an incredible way to use CBD to alleviate skin issues and to experience CBD without ingesting a capsule or oil.
CBD e liquids are an extremely popular method of using CBD. They are fast acting and provide relief at a rate very few other delivery methods can match.